Hobbs Medical Launches a Novel Aspiration Catheter with a Protective Membrane
The CleanCapture™ Aspiration Catheter was launched at ACG 2025
The device enables aspiration with minimal contamination. We hope this will optimize endoscopic aspiration for both clinical practices and microbiome research.”
STAFFORD SPRINGS, CT, UNITED STATES, December 16, 2025 /EINPresswire.com/ -- Hobbs Medical is proud to announce the launch of the CleanCapture™ Aspiration Catheter at ACG 2025. Dr. Ali Rezaie, MD, MSc, FRCP(C), Medical Director of the Cedars-Sinai GI Motility Program and Lead Developer of the CleanCapture™ device, and Brian Murray, General Manager at Hobbs Medical, Inc., attended the product debut (pictured).— Dr. Ali Rezaie, Medical Director of the Cedars-Sinai GI Motility Program
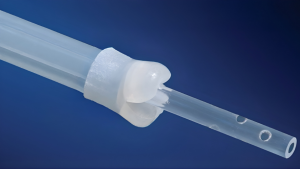
The CleanCapture™ Aspiration Catheter introduces a novel advancement in microbiome sampling. Engineered with a patent-pending protective membrane at the deployment tip, the device shields the aspiration channel during insertion and withdrawal, reducing contamination, which is a long-standing challenge in small-bowel aspirate collection.
Additional design elements, including a translucent outer sheath and a spiral hole pattern at the distal collection tip, further support efficient fluid retrieval. This first-of-its-kind catheter has the potential to advance diagnostic capabilities in SIBO research and broader GI microbiome analysis.
“The device enables aspiration with minimal contamination. We hope this will optimize endoscopic aspiration for both clinical practices and microbiome research,” said Dr. Rezaie.
“For over four decades, Hobbs Medical has manufactured and supplied quality devices for GI and pulmonary endoscopy procedures. We are grateful for the collaborative innovation that brought the CleanCapture™ and our other novel devices to market. We are excited for what these technologies will bring to the GI community,” said Murray.
For more information on the CleanCapture™ Aspiration Catheter, visit the Hobbs Medical CleanCapture™ page or contact Customer Service at (860) 684-5875 to place an order.
About Hobbs Medical
Hobbs Medical, headquartered in Stafford Springs, CT, is a privately held, family-owned global manufacturer and direct supplier of high-quality devices and accessories for flexible GI and pulmonary endoscopy.
In-stock inventory of standard endoscopic accessories includes (but is not limited to) soft plastic pancreatic and biliary stents, foreign body retrieval devices, balloon dilators, catheters, needles, brushes, and specialty items.
The company specializes in contract manufacturing and product development and prototyping. Hobbs Medical is ISO 13485 certified and registered with the Medical Device Single Audit Program (MDSAP).
Maria Contois
Hobbs Medical
+1 860-684-5875
mcontois@hobbsmedical.com
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.